Abstract
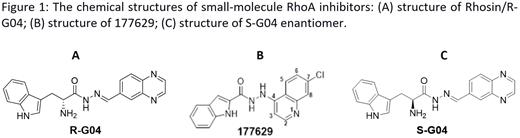
Platelet activation and aggregation play a key role in mediating hemostasis and thrombosis. The antiplatelet therapies currently available in the market are associated with a high risk of hemorrhage and are mostly irreversible in suppressing platelet activity; hence, there is a need to develop better therapeutic agents. Previous genetic and pharmacological studies have implicated the small GTPase RhoA in multiple platelet signaling pathways. We devised a lead RhoA activity-specific inhibitor, Rhosin/G04, based on the structure-function relationship of RhoA interaction with its activator, guanine nucleotide exchange factor (GEF) (Figure 1A). Rhosin/G04 binds to RhoA directly with micromolar affinity at a surface groove that is essential for GEF recognition and blocks GEF-mediated GTP loading to RhoA. Rhosin/G04 inhibits platelet spreading on fibrinogen and thrombin-induced platelet aggregation, mimicking effects of RhoA gene targeting. In the current work, we have utilized the inhibitory activity of G04 for platelet activation and its biochemical activity to define its structure-activity relationship (SAR) and to understand its mechanism of action in an effort to improve efficacy and druggability.
The structure of G04 in a groove of RhoA interaction was hypothesized based on the docking studies using Molsoft ICM-Pro. Cincinnati Children's Hospital Medical Center's compound library of over 360,000 chemicals was scanned for G04 analogs by similarity and substructure searches. In the initial screen, a human platelet aggregation assay was performed at both a low concentration (1 µg/ml) and a high concentration (5 µg/ml) of collagen. The first round similarity search resulted in a set of 7 compounds (Set-1), from which, compound 177629 showed significantly enhanced potency relative to G04 (Figure 1B). The second round of similarity searches for compounds more closely related to 177629 (Set-2) identified 14 compounds. The third-round search for other related compounds (Set-3) led to 9 additional compounds that add to the understanding of the SAR. The compounds that showed enhanced antiplatelet activity were examined for their potency and selectivity in in vitro biochemical binding assays and in suppressing RhoA-GTP formation and downstream phosphorylation of myosin light chain (p-MLC) signaling in platelets. The active compounds were further examined for their anti-platelet activities under diverse stimuli including thrombin, ADP, U46619 (a stable thromboxane receptor agonist), and arachidonic acid.
The most active compounds from Set-1, Set-2, and Set-3 inhibited platelet aggregation by at least 70% and showed IC 50 values below 6 µM. Of these compounds, 12 showed significantly greater potency than the initial compound, G04. The most active compounds were 177618, 177619, 177628, 177629, 177633, and 177634. These compounds specifically inhibited RhoA activity and blocked p-MLC. SAR analyses led us to believe that the quinoline is optimally attached to the hydrazine at the 4-position. The halogen (choloro- or trifluoromethyl-) substitution at the 7- or 8- position improved activity, and the 7- position may be slightly favored. The aryl group is considerably variable with similar potency between the indole, methylphenyl, and dichlorophenyl- groups.
Rhosin/G04 is the R enantiomer (i.e. Rhosin is R-G04), so its S enantiomer, S-G04 was also evaluated (Figure 1C). S-G04 is significantly more potent than R-G04 in inhibiting collagen-stimulated RhoA-GTP formation and aggregation of platelets, and its effect is completely reversible by washing the platelets. Finally, R-G04 and S-G04 showed differential inhibition of arachidonic acid and U46619 stimulated primary and secondary aggregation, highlighting the potential utilities of the inhibitors in dissecting different platelet activation mechanisms. S-G04 is active in inhibiting thrombin, ADP, U46619, and arachidonic acid-mediated platelet activation at submicromolar concentration, suggesting a broad role of RhoA signaling in integrating platelet signal cross talk.
In summary, evaluation of Rhosin/R-G04 analogs in a platelet activity screen identified a new generation of improved small-molecule RhoA inhibitors, including an enantiomer with significantly improved efficacy. These analog studies of novel anti-platelet agents provide a new approach to effectively and reversibly manipulate platelet activities.
No relevant conflicts of interest to declare.